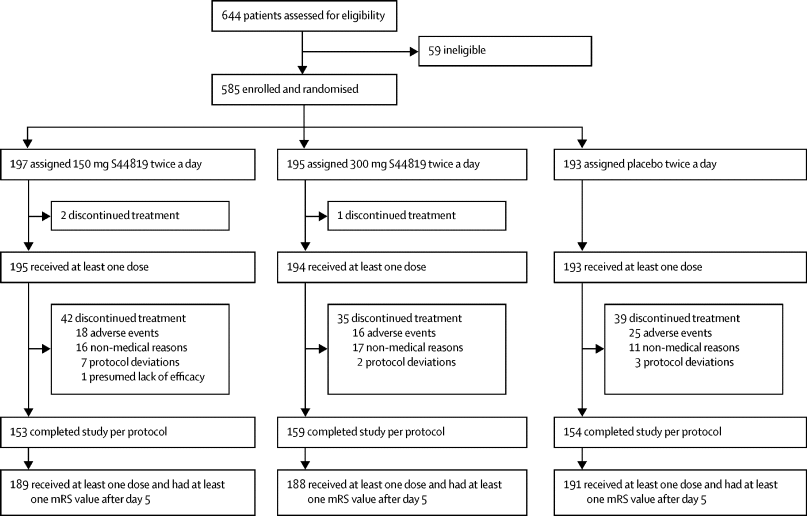
Curadev reports that the first patient dosed in a Phase 1a/b clinical trial of allosteric sting antagonist CRD3874-SI has completed the first treatment cycle.
PRNewswire
February 29, Boston, USA / Noida, Uttar Pradesh, India: Today, Curadev, a firm dedicated to drug discovery and development, announced a noteworthy advancement in its pipeline of immune-system-targeting cancer treatments.
An ongoing Phase 1a/b dose escalation and expansion clinical trial at Memorial Sloan Kettering Cancer Center (MSKCC) in New York is testing the intravenous infusion of Curadev’s systemic allosteric STING agonist, CRD3874-SI, in patients with advanced sarcoma and Merkel cell carcinoma.
Without any untoward incidents, the first patient’s four-week therapy cycle is over. Evaluating CRD3874-SI’s safety and tolerability as well as determining the recommended Phase 2 dosage (R2PD) and maximum tolerated dose (MTD) are the main goals of the dose escalation.
“This is a significant advancement for CRD3874-SI,” Curadev CEO and Chief Scientific Officer Dr. Arjun Surya stated. “It is the lead compound from an extensive small molecule drug discovery campaign that led to the discovery of our allosteric STING agonists which are clearly differentiated from agonists that bind to the orthosteric CDN site” .
Data presented at the Society for Immunotherapy of Cancer (SITC) annual meeting in November 2023 served as the foundation for the successful US-FDA application of CRD3874 to support FIH studies.
These included safety studies in non-human primates that supported the systemic distribution of CRD3874-SI via intravenous infusion in the clinic, as well as the single agent efficacy of CRD3874 administered intravenously in a variety of tumors seeded in human STING knock-in mice.
The evidence presented suggested that the unique pharmacological properties of CRD3874 may result from its allosteric binding mechanism, which simultaneously inhibits the proton channel-mediated toxicity and autophagy-driven effects of STING and enhances pro-inflammatory Type I IFN signaling. Cancers begin and spread because of their capacity to elude immune system destruction. The intention behind intravenous therapy
Principal Investigator Dr. Ciara Kelly, a specialist in treating and studying Merkel cell carcinomas and bone and soft tissue sarcomas, stated that CD3874-SI showed encouraging pre-clinical safety and anti-tumor activity.
It can be used to enhance both innate and adaptive immunity and has distinct qualities that set it apart from other STING agonists being studied. We are eager to assess this cutting-edge immunotherapeutic in a clinical context and give patients with advanced malignancies, such as sarcoma and Merkel cell carcinoma, this choice.”
“We are happy that Dr. Kelly and the knowledgeable team at MSKCC are examining the clinical efficacy of CRD3874-SI in the hopes of providing those who most require it with a potentially significant new treatment for advanced cancer.
“This first clinical trial of a compound discovered and developed by Curadev demonstrates our dedication to developing treatments that could change the course of cancer patients’ lives,” Dr. Surya continued.
Concerning Curadev
A biotech business at the trial stage called Curadev is committed to finding and creating new small molecule medicines to treat incurable illnesses.
With the help of cutting-edge screening techniques, conventional wet-lab chemistry and pharmacology, and a target-centric strategy, Curadev has amassed an exceptional portfolio of discovery research projects that have produced patent-protected drug candidates that regulate next-generation therapeutic targets. Go to curadev.in to find out more about Curadev.